Chemical indicators are easy, are cheap, and indicate the merchandise has become exposed to the sterilization process. In a single research, chemical indicators were more possible than biological indicators to inaccurately suggest sterilization at marginal sterilization periods (e.g., 2 minutes)847. Chemical indicators ought to be made use of in conjunction with biological indicators, but determined by recent experiments must not swap them as they suggest sterilization at marginal sterilization time and because merely a biological indicator consisting of resistant spores can evaluate the microbial killing ability with the sterilization method.847, 974. Chemical indicators are affixed on the outside of each and every pack to indicate that the package deal continues to be processed by way of a sterilization cycle, but these indicators will not verify sterilization continues to be attained.
SciCan’s progressive G4 Technological innovation, now WiFi enabled, routinely records and screens each and every cycle 24/seven And so the tedious task of manually logging cycle data can now be carried out routinely and mistake-no cost.
Biological indicators are acknowledged by most authorities as remaining closest to the ideal monitors in the sterilization system 974, 975 because they measure the sterilization procedure specifically by using the most resistant microorganisms (i.e., Bacillus
Time, Temperature and Stress must be exactly managed once and for all sterilization outcomes The Gravity Cycle is The only cycle; perfect for sterilizing liquids, media, glassware & plastic, society plates and unwrapped instruments.
This may be analyzed by passing the steam-air mixture liberated from your discharge faucet right into a pail of water through a connecting rubber tube. If the air bubbles cease coming while in the pail, it indicates that every one the air has long been displaced by steam.
When 3 consecutive cycles clearly show negative biological indicators and chemical indicators with an accurate conclusion issue response, you could set the change made into plan use811-814, sterilization in pharma 958. Merchandise processed throughout the three evaluation cycles must be quarantined right until the examination final results are detrimental.
three. Exhaust Phase: Strain is introduced with the chamber, but temperatures keep on being quite higher. Experts should really just take treatment when removing warm contents with the autoclave.
Rubbers are a wonderful materials for forming seals and closures. A closure is a component in the bundle utilized for… Examine more: Rubber as a fabric for seals and closures
SciCan’s innovative G4 Technological know-how, now WiFi enabled, routinely information and displays each and every cycle 24/seven Therefore the wearisome endeavor of manually logging cycle facts can now be finished immediately and mistake-free of charge.
The Biosealer® TC offers a extensive twenty mm seal for more robust disconnection. Additionally, a cutting guideline embedded in the seal assures operators could make a clear Minimize with scissors.
What's more, There's prospect of transmission of an infection from patient to client; from affected individual or to wellness care staff, and vice versa; or in the ecosystem into the patient throughout the incorrect sterilized or disinfected products. For this reason, clinical staff, laboratory men and women and the wellbeing care vendors should have much better knowledge with regards to these tactics to circumvent the unfold of those pathogens.
Wrapping objects in aluminum foil is just not advisable as it may possibly interfere with steam penetration. Article content must be wrapped in products that enable steam penetration.
The chance of transmission of an infection with these items is noticed to be the bottom. Having said that, they add for the transmission of an infection in indirect way. For example, methicillin-resistant Staphylococcus aureus
Biological and chemical indicator tests is likewise carried out for ongoing high quality assurance tests of agent samples of true products and solutions becoming sterilized and product or service tests when main modifications are created in packaging, wraps, or load configuration. Organic and chemical indicators are positioned in merchandise, which happen to be read more processed in an entire load.
 Barret Oliver Then & Now!
Barret Oliver Then & Now!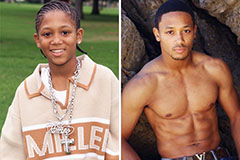 Romeo Miller Then & Now!
Romeo Miller Then & Now! Michael C. Maronna Then & Now!
Michael C. Maronna Then & Now! Andrew McCarthy Then & Now!
Andrew McCarthy Then & Now! The Olsen Twins Then & Now!
The Olsen Twins Then & Now!